Abstract
Background: Hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA; also known as TA-TMA) is a potentially life-threatening complication associated with multi-organ injury and significant morbidity and mortality. HSCT-TMA incidence rates of up to 39% have been reported in both pediatric and adult allogeneic HSCT (alloHSCT) recipients; high-risk pediatric patients with proteinuria and evidence of terminal complement activation (elevated sC5b-9) had a 1-year post-HSCT survival of <20% versus 100% in those with normal sC5b-9 levels and no proteinuria (Jodele S, et al. Blood. 2014;124:645-653). In the HSCT setting, toxic conditioning regimens, infection, and graft-versus-host disease (GVHD) can cause endothelial injury, which triggers activation of the lectin pathway of complement and in turn the coagulation cascade, together leading to TMA. Narsoplimab (OMS721) is a fully human IgG4 monoclonal antibody that inhibits mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway and an activator of the coagulation cascade. Narsoplimab was previously evaluated for safety and efficacy in adults with high-risk HSCT-TMA in an open-label pivotal trial (NCT02222545); narsoplimab treatment was well tolerated and resulted in clinical response and favorable overall survival (Khaled SK, et al. J Clin Oncol. 2022; JCO2102389).
Currently, HSCT-TMA has no approved therapy, and therefore represents a significant unmet medical need. Here, we describe the design of the first clinical trial evaluating the efficacy and safety of narsoplimab in pediatric patients with HSCT-TMA.
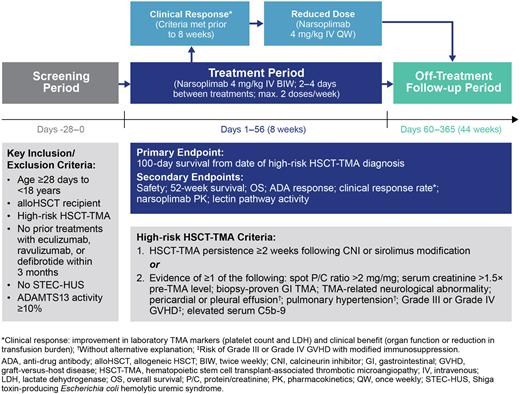
Study Design and Methods: This is an open-label, multi-center Phase 2 trial of narsoplimab in pediatric patients (Figure 1). Eligible patients are aged ≥28 days to <18 years, have received alloHSCT for the treatment of non-malignant or malignant disease, and have been diagnosed with high-risk HSCT-TMA. Exclusion criteria include prior treatment with eculizumab, ravulizumab, or defibrotide within 3 months prior to screening, having Shiga toxin-producing Escherichia coli hemolytic uremic syndrome (STEC-HUS), or ADAMTS13 activity <10%.
Patients will receive narsoplimab 4 mg/kg via intravenous (IV) infusion twice weekly during the 8-week treatment period (Days 1-56). If a patient meets all clinical response criteria prior to 8 weeks of treatment, dosing may be decreased to 4 mg/kg IV once weekly until the end of the treatment period. Narsoplimab can be used in conjunction with standard-of-care treatments. At the end of the treatment period, patients will enter a 44-week follow-up period (Days 60-365). The planned recruitment is at least 18 pediatric patients, distributed across the age range.
The primary endpoint is 100-day survival from date of HSCT-TMA diagnosis. Secondary endpoints include: safety, survival at 52 weeks, overall survival from date of HSCT-TMA diagnosis, anti-drug antibody response, clinical response rate, narsoplimab pharmacokinetics and measurement of lectin pathway activity.
Conclusion: Following the favorable results obtained in a pivotal Phase 2 study in adults with HSCT-TMA, further evaluation of narsoplimab efficacy and safety in pediatric patients with high-risk HSCT-TMA is warranted.
Disclosures
Pullman:Omeros Corporation: Consultancy, Current equity holder in publicly-traded company. Brough:Omeros Corporation: Current Employment, Current equity holder in publicly-traded company. Soto:Omeros Corporation: Current Employment, Current equity holder in publicly-traded company. Meng:Omeros Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal